By Deanna M. Soper, PhD
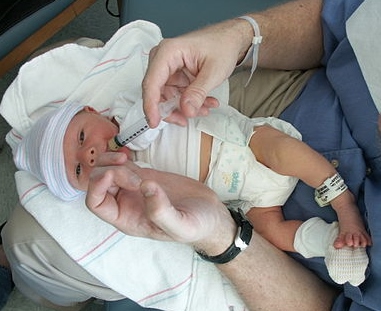
What is antenatal (prenatal) milk expression? Why would women do it? Antenatal milk expression (AME) refers to extracting colostrum (the first milk) from the breast prior to birth, usually by hand expressing. In recent years, AME has been suggested to some mothers who have Type I or gestational diabetes. Babies born to mothers with diabetes may be at an increased risk of being hypoglycemic (low blood sugar) at birth and are sometimes supplemented with formula in an attempt to increase their glucose levels. However, formula supplementation, particularly early on, can have devastating effects on breastfeeding success. In addition, formula supplementation – even just one bottle – can carry health risks for the infant.(1)
For these reasons, some healthcare providers are now suggesting that mothers with Type I, or gestational diabetes, express their colostrum before their babies are born. This has triggered a hot debate raising many questions surrounding safety, efficacy, and whether there are nutritional differences between antenatally expressed colostrum and the colostrum that is produced post birth.
Historical Perspective
Harold Waller conducted some of the earliest studies of antenatal milk expression. He was mostly interested in understanding why long-term breastfeeding failed during a time when little was known about how lactation was maintained in humans. He used AME to determine if teaching mothers hand expression techniques prenatally improved breastfeeding rates.(2,3) Articles on AME from Waller’s time until post-2000 are scant, but those that exist focus on AME as a method to prepare women to breastfeed, and the expressed colostrum was discarded.(4) However, today AME is more focused on collecting the colostrum prenatally for supplementing infant feeding, particularly for babies who are at risk of hypoglycemia at the time of birth.(4)
Potential Benefits
There may be many reasons that antenatally expressed milk may be beneficial. They include reduction in the use of formula, increase in breastfeeding rates, and additional nutritional and immunologic protection. Formula supplementation occurs for a variety of reasons(5,6), but if pre-expressed colostrum was readily available, formula use may diminish. Mothers with Type I and gestational diabetes often have babies who are supplemented with formula due to unstable infant glucose levels at birth(7). However, in many cases these supplemental feeds may be unnecessary, as the tests used to determine infant glucose levels can be quite unreliable (8,9). Late onset of lactogenesis II (mature milk production) occurs frequently in mothers with Type I and gestational diabetes. This outcome may be hormonally influenced(7), but could also be caused by not breastfeeding or expressing frequently enough, which can be the result of formula supplementation.(10)
Diabetic mothers may not be the only group who could benefit from antenatal milk expression. Expectant mothers who are known to have insufficient breast tissue, polycystic ovarian disease, multiple sclerosis, or those who have undergone breast surgery, may all benefit from AME. (7) In addition, mothers who have medical concerns about early milk production may benefit from AME.
Breastfeeding success is important for the mother, given the fact that it is known to lower blood pressure, decrease the risk of premenopausal breast cancer, decrease the risk of ovarian cancer, provide protection against osteoporosis, and assist in losing weight gained from the pregnancy.(11,17) However, with the known risks of formula feeding and the importance of ingesting colostrum and mature breast milk, breastfeeding may be even more critical for an infant.(18,20) Colostrum is considered the “early milk,” can exhibit a wide range of thickness and color, and has the right mix of minerals, vitamins, proteins, and fats for newborns.(21) Ingesting this “early milk” has many effects on the newborn body. It acts as a laxative to assist the newborn in expelling meconium, the first dark tarry stools from the digestive tract. In addition, it is a living culture of cells that provide immunization and protection against bacteria and viruses that the newborn encounters.(18) During the third trimester of pregnancy, the breasts begin to produce colostrum. Some women leak this fluid and choose to collect and freeze it to feed to the newborn, if necessary. However, no studies so far have determined how prenatal and postnatal colostrum might differ in both nutritional and cellular content.
It must be noted that many of the studies that involve the use of antenatal milk expression have low sample sizes or inadequate experimental design.(22) Thus, the safety and efficacy of antenatal milk expression for the purposes of retaining colostrum or preparing for breastfeeding have not been thoroughly evaluated. For more discussion on this topic see(23).
Concerns with antenatal milk expression
Antenatal milk expression is usually suggested to start between gestational weeks 34 and 37 (7,24). Because nipple stimulation can lead to oxytocin release and oxytocin is known to play a role in cervical ripening and induction of labor, one concern is the possibility of inducing labor too early. One recent study has indicated that infants of mothers who have practiced AME have lower birth weights and shorter gestation time, which may mean that nipple stimulation during AME caused cervical ripening and labor induction prematurely (25). Those attempting to interpret these results should note that this study had low sample sizes and restricted participants to mothers with diabetes, which means that generalization of these results to non-diabetic mothers would be inappropriate. Another measure that can indicate whether AME may lead to increased risk of premature labor is number of infant admissions to special care units. Two recent studies have found that infants born to mothers who have antenatally expressed milk may have an increased risk of admission to special care units (26,27), although both studies were flawed to some degree. Soltani (2008) was not formally published in a peer-reviewed journal, and thus a complete analysis of experimental design is not possible. In addition, it should be noted that Soltani (2008) recognized that low sample size of the study made data interpretation difficult. Forster (2009) also had low sample sizes and questionable experimental design. For more discussion and an in-depth analysis of Forester (2009) methodology, see Chapmen (2012).
Ultimately, given the lack of reliable studies, it is difficult to determine if AME would actually lead to early initiation of labor that may result in low birth weight or increased risk of admission of the infant to a special care unit. Certainly, more studies about labor initiation should be undertaken. However, several questions come to mind such as: How much oxytocin is released during nipple stimulation? How does the level of oxytocin release during AME compare to other behaviors the pregnant human female exhibits such as kissing, orgasm, cuddling with other children or her partner? If nipple stimulation during AME causes oxytocin release that results in premature labor, are mothers who are currently nursing a toddler while in their third trimester at an increased risk of premature labor? Will oxytocin spikes from any of these behaviors (i.e. AME, orgasm, nursing a toddler) cause early onset of labor?
Unfortunately, few studies that attempt to answer such questions exist. Surprisingly, scientists still do not fully understand what causes the onset of labor. However, it is known that several physiologic, anatomical, and hormonal changes occur prior to the onset of labor, indicating that induction of labor is a complex process that involves not only oxytocin release, but also a host of other biologic events (28-30). In addition, oxytocin is known to elevate during sexual behavior in multiple different species (31), and in the human female can elevate significantly above baseline after stimulation through orgasm (32). One behavior, nipple stimulation, may occur through sexual contact or when a mother is pregnant and still nursing another child. However, oxytocin release from nipple stimulation is significantly less in the pregnant woman compared to the non-pregnant state (33). In at least one study, pregnant nursing mothers have not been found to be at an increased risk of preterm labor (34). Given this information, it seems that nipple stimulation, as the result of AME, is unlikely to cause early onset labor. At the same time, each woman is unique. If she chooses to engage in AME, she should be aware of the signs of preterm labor, especially if she experienced preterm labor in a previous pregnancy, and should discuss the safety of this practice and any other concerns with her care provider prior to initiation.
A mother who is considering antenatal expression needs to investigate this topic fully to determine if it right for her individual situation. A mother who chooses to antenatally express milk and plans to bring it to the hospital should talk to her care provider and hospital prior to birth as they may not have encountered this frequently before. A check with one hospital in a suburb of Chicago revealed that no protocols exist for antenatal milk expression or storage. A few hospital protocols from Australia and New Zealand are online (See Links 1 and 2). Antenatally expressed milk needs to be placed in collecting tubes and frozen(35,36), unless the mother is going to be induced or have a planned cesarean birth within a day or two. Then the colostrum can be safely kept at room temperature up to 24 hours(37,38) or kept in the refrigerator for up to 8 days.(39,40) Ideally, the colostrum would be used within 72 hours.(35,41,42) If the mother has frozen colostrum, she will need to have access to a freezer upon arrival at her birthing location. In addition, syringes, cups or spoons should be available for use to feed the baby the colostrum because of the small quantities and the potential for early bottle-feeding to interfere with breastfeeding. Talking to the hospital’s International Board Certified Lactation Consultant (IBCLC), if one is on staff, prior to giving birth may be beneficial to determine if the hospital is equipped with necessary supplies or if a mother will be required to bring her own (i.e collecting tubes, pumping supplies).
Expression of milk prior to birth may be beneficial for some, but is not required by all. Today, mothers who know they may need extra colostrum at birth may use this practice. Although more research is needed to better evaluate the safety, efficacy, and benefits of AME, existing published studies can give us insights into the possibility for this practice to positively influence breastfeeding rates and reduce formula supplementation.
Literature Cited
1. Walker, M. (2004). Just One Bottle Won’t Hurt – or Will It? Retrieved March 11, 2013
2. Waller, H. (1946). The early failure of breast feeding: A clinical study of its causes and their prevention. Archives of Disease in Childhood, 21(105), 1-12.
3. Waller, H. (1950). The early yield of human milk, and its relation to the security of lactation. Lancet, 53-56.
4. Chapman, T. (2012). Antenatal breast expression: A critical review of the literature. Midwifery, doi: 10.1016/j.midw.2011.12.013.
5. Gagnon, A. J., Leduc, G., Waghorn, K., Yang, H., & Platt, R. W. (2005). In-hospital formula supplementation of healthy breastfeeding newborns. Journal of Human Lactation, 21(4), 397-405.
6. Tender, J. A. F., Janakiram, J., Arce, E., Mason, R., Jordan, T., Marsh, J., . . . Moon, R. Y. (2009). Reasons for in-hospital formula supplementation of breastfed infants from low-income families. Journal of Human Lactation, 25(1), 11-17.
7. Cox, S. G. (2006). Expressing and storing colostrum antenatally for use in the newborn period. Breastfeeding Review, 14(3), 11-16.
8. Cornblath, M., Hawdon, J. M., Williams, A. F., Aynsley-Green, A., Ward-Platt, M. P., Schwartz, R., & Kalhan, S. C. (2000). Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics, 105(5), 1141-1145.
9. Hawdon, J. M. (2005). Blood glucose levels in infancy-clinical significance and accurate measurement. Infant, 2(2), 24-27.
10. Wight, N., & Marinelli, K. A. (2006). ABM Clinical Protocol #1: Guidelines for Glucose Monitoring and Treatment of Hypoglycemia in Breastfed Neonates. Breastfeeding Medicine, 1(3), 178-184.
11. Baker, J. L., Gamborg, M., Heitmann, B. L., Lissner, L., Sørensen, T. I. A., & Rasmussen, K. M. (2008). Breastfeeding Reduces Postpartum Weight Retention. American Journal of Clinical Nutrition, 88(6), 1543-1551.
12. Becher, H., Schmidt, S., & Chang-Claude, J. (2003). Reproductive factors and familial predisposition for breast cancer by age 50 years. A case-control-family study for assessing main effects and possible gene-environment interaction. International Journal of Epidemiology, 32(1), 38-48.
13. Carranza-Lira, S., & Mera Paz, J. (2002). Influence of number of pregnancies and total breast-feeding time on bone mineral desnity. International Journal of Fertility and Women’s Medicine, 47(4), 169-171.
14. Paton, L. M., Alexander, J. L., Nowson, C. A., Margerison, C., Frame, M. G., Kaymakci, B., & Wark, J. D. (2003). Pregnancy and lactation have no long-term deleterious effect on measures of bone mineral in healthy women: a twin study. The American Journal of Clinical Nutrition, 77(3), 707-714.
15. Rosenblatt, K. A., & Thomas, D. B. (1993). Lactation and the risk of epithelial ovarian cancer. International Journal of Epidemiology, 22(2), 192-197.
16. Yen, M., Yen, B. L., Bai, C., & Lin, R. S. (2003). Risk factors for ovarian cancer in Taiwan: a case-control study in a low-incidence population. Gynecologic Oncology, 89(2), 318-324.
17. Zheng, T., Duan, L., Liu, Y., Zhang, B., Wang, Y., Chen, Y., . . . Owens, P. H. (2000). Lactation reduces breast cancer risk in Shandong Province, China. American Journal of Epidemiology, 152(12), 1129-1135.
18. Hanson, L. A. (2007). Session 1: Feeding and infant development breast-feeding and immune function. Proceedings of the Nutrition Society, 66(3), 384-396.
19. Luopajärvi, K., Savilahti, E., Virtanen, S. M., Ilonen, J., Knip, M., Åkerblom, H. K., & Vaarala, O. (2008). Enhanced levels of cow’s milk antibodies in infancy in children who develop type 1 diabetes later in childhood. Pediatric Diabetes, 9(5), 434-441.
20. Tiittanen, M., Paronen, J., Savilahti, E., Virtanen, S. M., Ilonen, J., Knip, M., . . . Vaarala, O. (2006). Dietary insulin as an immunogen and tolerogen. Pediatric Allergy and Immunology, 17(7), 538-543.
21. Lauwers, J., & Swisher, A. (2011). Counseling the Nursing Mother A Lactation Consultant’s Guide (5 ed.). Sudbury, MA: Jones & Bartlett Learning.
22. Chapman, T. (2012). Antenatal breast expression: Exploration and extent of teaching practices amongst International Board Certified Lactation Consultant midwives across Australia. Women Birth, doi:10.1016/j.wombi.2012.01.001. doi: 10.1016/j.wombi.2012.01.001
23. Cox, S. G. (2010). An ethical dilemma: should recommending antenatal expressing and storing of colostrum continue? Breastfeeding Review, 18(3), 5-7.
24. Service, W. S. H. (2012, June 2012). Antenatal Milk Expressing Retrieved January 31, 2013
25. Soltani, H., & Scott, A. M. S. (2012). Antenatal breast expression in women with diabetes: outcomes from a retroscpective cohort study. International Breastfeeding Journal, 7(18), 1-5.
26. Forster, D., McEgan, K., Ford, R., Moorhead, A., Opie, G., Walker, S., & McNamara, C. (2009). Diabetes and antenatal milk expressing: a pilot project to inform the development of a randomised controlled trial. Midwifery, 2, 209-214.
27. Soltani, H. (2008). Antenatal breast expression and the risk of labour induction: a study in a baby friendly hospital. Paper presented at the The International Confederation of Midwives Conference, Glasgow.
28. Garfield, R. E., Saade, G., Buhimschi, C., Buhimschi, I., Shi, L., Shi, S. Q., & Chwalisz, K. (1998). Control and assessment of the uterus and cervix during pregnancy and labour. Human Reproduction Update, 4(5), 673-695.
29. Gimpl, G., & Fahrenholz, F. (2001). The Oxytocin Receptor System: Structure. Function, and Regulation Physiological Reviews, 81(2), 629-683.
30. Castracane, V. D. (2000). Endocrinology of preterm labor. Clinical Obstetrics and Gynecology, 43(4), 717-726.
31. Carter, C. S. (1992). Oxytocin and sexual behavior. Neuroscience & Biobehavioral Reviews, 16(2), 131-144.
32. Carmichael, M. S., Warburton, V. L., Dixen, J., & Davidson, J. M. (1994). Relationships among cardiovascular, muscular, and oxytocin responses during human sexual activity. Archives of Sexual Behavior, 23(1), 59-79.
33. Amico, J. A., & Finley, B. E. (2008). Breast stimulation in cycling women, pregnant women and a woman with induced lactation: pattern of release of oxytocin, prolactin and luteinizing hormone. Clinical Endocrinology, 25(2), 97-106.
34. Moscone, S. R., & Moore, M. J. (1993). Breastfeeding during pregnancy. Journal of Human Lactation, 9(2), 83-88.
35. RamÍrez-Santana, C., Pérez-Cano, F. J., Audí, C., Castell, M., Moretones, M. G., López-Sabater, M. C., . . . Franch, A. (2012). Effects of cooling and freezing storage on the stability of bioactive factors in human colostrum. Journal of Dairy Science, 95(5), 2319-2325.
36. Takci, S., Gulmez, D., Yigit, S., Dogan, O., Dik, K., & Hascelik, G. (2012). Effects of freezing on the bactericidal activity of human milk. Journal of Pediatric Gastroenterology and Nutrition, 55(2), 146-149.
37. Nwankwo, M. U., Offor, E., Okolo, A. A., & Omene, J. A. (1988). Bacterial growth in expressed breast-milk. Annals of Tropical Pediatrics, 8(2), 92-95.
38. Pittard III, W. B., Anderson, D. M., Cerutti, E. R., & Boxerbaum, B. (1985). Bacteriostatic qualities of human milk. The Journal of Pediatrics, 107(2), 240-243.
39. Ogundele, M. O. (2002). Effects of storage on the physicochemical and antibacterial properties of human milk. British Journal of Biomedical Science, 59(4), 205-211.
40. Pardou, A., Serruys, E., Mascart-Lemone, F., Dramaix, M., & Vis, H. L. (2009). Human milk banking: influence of storage processes and of bacterial contamination on some milk constituents. Neonatology, 65(5), 302-309.
41. Igumbor, E. O., Mukura, R. D., Makandiramba, B., & Chihota, V. (2000). Storage of breast milk: effect of temperature and storage duration on microbial growth. The Central African Journal of Medicine, 46(9), 247-251.
42. Silvestre, D., Lopez, M. C., March, L., Plaza, A., & Martinez-Costa, C. (2006). Bactericidal activity of human milk: stability during storage. British Journal of Biomedical Science, 63(2), 59-62.
Deanna M. Soper, PhD
“Dr. Soper received her Ph.D. in Ecology, Evolution, and Behavior from Indiana University and is currently a Postdoctoral Researcher at the University of Iowa. She is also in the process of becoming a Breastfeeding USA Counselor and is nursing her 22 mon
© Copyright Breastfeeding USA 2013. All rights are reserved.
